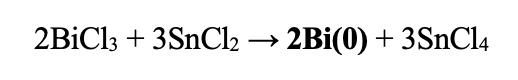
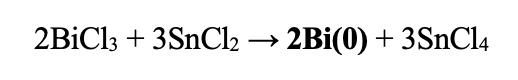

| Redox Potentials: | SnCl2 → SnCl4 | -0.1375 | |
| Bi3+ + 2e- → Bi | 0.308 | ||
| Total: | 0.1705 |
There is no need to prepare the glassware before intercalation. Setup the reflux setup as shown. There is also no need to use an air-free setup. Prepare a stock solution of stannous chloride from 0.01 – 0.02 g of SnCl2 in 5 mL.
Heat a solution of 0.016 g bismuth(III) chloride in 5 mL acetone in the round-bottom flask to just below reflux (48°C). Once heated, drop the 2D material, free or suspended on a substrate, into the round bottom flask. Dropwise, add in the solution of tin(II) chloride very slowly over the course of 3 hours. Keep heating during this time at just below reflux. After 3 hours, and complete injection of the stannous chloride solution, wait an additional 30 – 45 mins with heating. After, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetone (~45°C) will help remove any other salt impurities that may end up on the substrate.
| Bi(III)Cl3 | SnCl2 | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|---|
| 0.016 g | 0.1 g | 5 mL | 3.75 hours | ?? atm % ± 5% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)