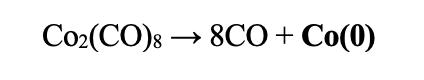
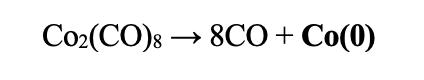
Easy Method: With a lot of layered materials, it is posible to just intercalate Co by imersion. Under inert atmosphere, such as in a glovebox, immerse the crystal in a solution of 0.03g of cobalt carbonyl in 6mL of acetone. Let the solution and crystals sit capped for 24-48 hours. The solution will be nearly transparent afterwards. Remove the crystals. Rinse with acetone and/or ethanol 3 times. Dry.
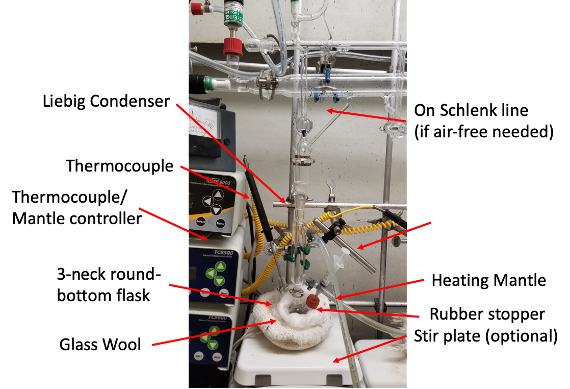
Published Method: Setup the reaction as according to Fig 3. Place the 2D material in the round-bottom flask, evacuate and flushed with N2 gas. In a glovebox, create two stock solutions. Stock solution 1 should be 5 mL of anhydrous acetone. Stock solution 2 should be created from 0.01-0.03 g of dicobalt octacarbonyl dissolved in 3-5mL of air free acetone, respectively. Inject the first stock solution of anhydrous acetone into the round-bottom flask and heat to just below reflux (~45-48°C). Dropwise, inject in the cobalt solution 2 over the course of 1.5 hours. After all solution has been injected, allow the mixture to sit just below reflux for 1 more hour. Remove substrates or material from the solution and rinsed with hot acetone and hot ethanol, exposing to air at this point is okay.
Ideally, and if the reaction is done to perfection, the solution will resemble a beautiful purple gel. This gel will keep the excess cobalt long enough to remove the substrate. After removal, the gel will crash out a black cobalt precipitate. If things didn’t go quite ideally, there may be some black cobalt metal nanoparticles generated during reaction. These will precipitate out on the flask but may end up on the surface of the 2D material or substrate. These particles are removed by rinsing with ethanol and acetone (25°C) and/or via sonication of the crystals.
If you are really lazy, you can avoid doing this reaction dropwise. Just mix up 0.03 g cobalt carbonyl in 8 mL of anhydrous acetone. Inject it into your sample in a round bottom flask on the Schlenk line and heat it up under nitrogen to 30 C for four hours. This seems to work okay though does not have as much cobalt intercalated as the dropwise route.
| Co2(CO)8 | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|
| 0.01 g | Total = 8 mLs | 2.5 hours | 20-30 atm % ± 3% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)