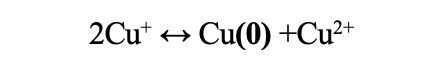
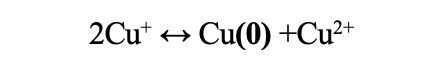

Prepare the glassware by letting sit overnight in DI water. Test the pH of the DI water; pH should be about ~ 5.5-6.5. After 24 hours, dry the glassware in a drying oven (or overnight on a rack) for an additional 24 hours. These steps are critical. Without it, the pH may be too basic forcing more Cu2+ to form over Cu(0). See the Pourbaix diagram for copper in water. The ideal region for pH is the blue box. Be aware, this diagram is for copper in an aqueous solution (boxes not identified are oxide phases) but it serves as a fairly accurate guide for intercalation in acetone.
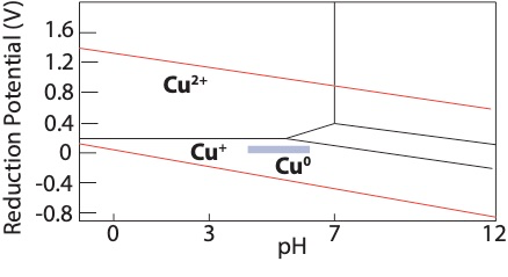
Pourbaix Diagram of copper in water. Blue is the ideal pH region for copper intercalation
The tetrakisacetonitrile copper hexafluorophosphate precursor powder should be a white to yellow color. Bluish powder means that your source has been exposed to too much water and should not be used.
Setup the reflux apparatus as shown in Fig 1. There is no need to use an air-free setup. Heat a solution of 0.01 g tetrakisacetonitrile copper hexafluorophosphate in 5 mL acetone in the round-bottom flask to just below reflux (48°C). Once heated, drop in the 2D material, free or suspended on a substrate, into the round bottom flask. Keep heating just below reflux for 4 hours. After 4 hours, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetone will help remove any other salt impurities that may end up on the substrate.
The reaction solution should be a light yellow color with a tiny amount of whitish precipitate after 4 hours. A blue color means that the solution was too basic during the reaction and too much Cu2+ was formed and not enough Cu(0). If the solution is allowed to sit and cooled to room temperature after the intercalation reaction, the solution should develop a yellow/white/blue powder precipitate (which is a copper – and sometimes contaminated with Si from the glass - PF6 compound). With time, a solution allowed to sit will turn blue. This is the Cu2+ coming out of its solvent shell and not complexed with the PF6. The copper 2+ during the reaction will be solvated in a solvent shell as shown in the example with acetone below.
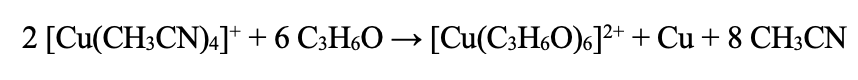
Things to be aware of:
- The substrate matters. A silicon substrate without much of an oxide layer can mediate electroless deposition of copper. This will be seen as dendritic looking copper on the substrate. If this shows up, the intercalation reaction was unsuccessful.
- If the reaction solution is turning bright blue within ten minutes of starting, there are ways to save it. Add a tiny drop (uL or less) of nitric acid to the reaction. Note that this is adding an acid to an organic which generates a lot of hydrogen! This can be very dangerous so use this only if desperate. If it turns blue after an hour or so, don’t worry too much about it.
- Make sure to prep the glassware and check the pH before proceeding. This is pretty much the one thing that can kill the reaction.
| Cu+1 | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|
| 0.14 g | 5 mL | 10 min | 30-50 atm % ± 20% |
| 0.07 g | 5 mL | 10 min | 20-40 atm % ± 20% |
| 0.01 g | 5 mL | 10 min | 10 atm % ± 10% |
| 0.01 g | 5 mL | 4 hr | 60 atm % ± 5% |
| 0.01 g | 5 mL | 30 min | 25-40 atm % ± 15% |
| 0.01 g | 5 mL | 10 min | 5-10 atm % ± 5% |
Note that with 0.14 g tetrakisacetonitrile copper hexafluorophosphate in 5 mL acetone, this intercalation reaction can be done in about 10 mins with a large distribution of intercalant.
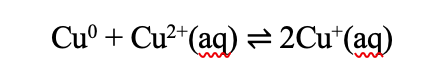
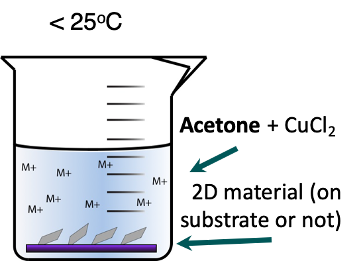
Setup for Deintercalation.
Deintercalation without exchange can be performed by placing the substrate in a round bottom flask or beaker followed by addition of a solution of 0.001 g CuCl2 dissolved in 0.5 ml acetone with 10 ml of trioctylphosphine. Keep the substrate in solution at room temperature for one hour. Remove and rinse with acetone. Cation exchange between Cu2+ and Ge2+, Mo4+, W4+, In3+, Nb4+ in acetone is not favored so the deintercalation of Cu in MoS2, BN, graphite and other layered chalcogenides does not require addition of a soft base.
M. Wang, I. Al-Dhahir, J. Appiah, and K. J. Koski, Deintercalation of zero-valent metals from 2D layered chalcogenides. Chem. Mater. 29, 1650-1655 (2017).