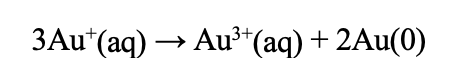
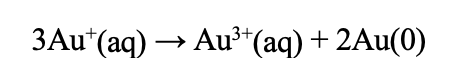

Use whatever dissolves best. Gold (I) chloride is hard to dissolve in acetone, but you can dissolve it in water and have a slightly cheaper intercalation procedure.
If using gold (I) chloride, this intercalation route works best using anhydrous acetone and performing the reaction under inert atmosphere as it is difficult to get some salts to dissolve. Most of the other gold salts mentioned above are also air sensitive and require air-free techniques.
This requires preparation of the glassware before intercalation. Prepare the glassware to achieve a pH of ~7 by letting sit overnight in water (not distilled) or in a base bath. Test the pH of the water; pH should be about 7. After 24 hours, dry the glassware in a drying oven (or overnight on a rack) for an additional 24 hours. These steps are somewhat critical for gold. Without it, the pH may be too acidic forcing more Au2+ to form over Au(0). This can be seen in the Pourbaix diagram below. The ideal region is the blue box. There is a lot of room for error for achieving the correct pH for gold intercalation. Be aware, this diagram is for gold in an aqueous solution but it serves as a fairly accurate guide for intercalation in acetone.
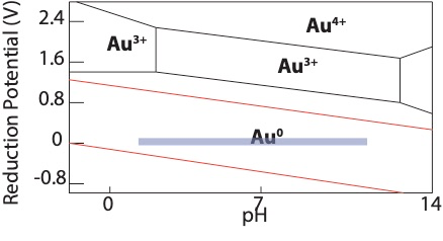
Pourbaix Diagram of gold in water. Blue is the ideal pH region for gold intercalation
Setup the reflux setup shown. There is no need to use an air-free setup unless the chosen Au(I) salt is not air-stable. Heat a solution of 0.01 g gold(I) chloride in 5 mL acetone in the round-bottom flask to just below reflux (48°C). Once heated, drop the 2D material, free or suspended on a substrate, into the round bottom flask. Keep heating just below reflux for 4 hours. After 4 hours, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetone will help remove any other salt impurities that may end up on the substrate.
| AuCl | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|
| 0.01 g | 5 mL | 4 hours | 20 atm % ± 5% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)