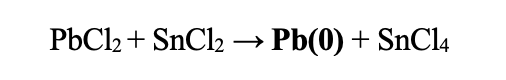
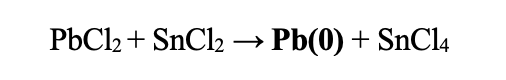

| Redox Potentials: | SnCl2 → SnCl4 | -0.1375 | |
| Pb2+ + 2e- → Pb(0) | -0.1262 | ||
| Total: | 0.0113 |
This reaction will favor formation of Pb(0) and SnCl4. Make sure to use dilute amounts of lead salt as it will reduce quickly and can deposite. The more dilute, the better.
There is no need to prepare the glassware before intercalation. Setup the reflux apparatus as shown in Fig 2. There is also no need to use an air-free setup. Prepare a stock solution of stannous chloride of 0.014 g of SnCl2 in 5 mL.
Heat a solution of 0.014 g lead(II) chloride in 5 mL acetone in the round-bottom flask (above) to just below reflux (48°C). Once heated, drop the 2D material, free or suspended on a substrate, into the round bottom flask. Dropwise, add in the solution of tin(II) chloride very slowly over the course of 3 hours. Keep heating during this time at just below reflux. After 3 hours, and complete injection of the stannous chloride solution, wait an additional 30 – 45 mins with heating. After, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetone (~45°C) will help remove any other salt impurities that may end up on the substrate.
| PbCl2 | SnCl2 | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|---|
| 0.014 g | 0.01 g | 10 mL | 3 hours | 1 atm % ± 1% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)