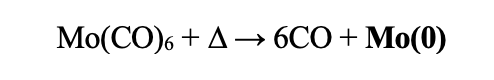
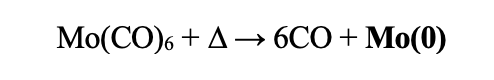
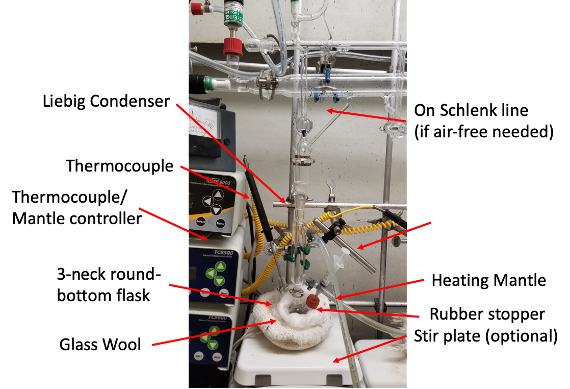
Setup the reaction according to Fig 3. Place the 2D material in the round-bottom flask, evacuate and flushed with N2 gas. In a glovebox, create two stock solutions. Stock solution 1 should be 5 mL of anhydrous acetone. Stock solution 2 should be created from 0.01-0.03 g of moly carbonyl dissolved in 5mL of air free acetone. Inject stock solution 1 of anhydrous acetone into the round-bottom flask and heat to just below reflux (~45-48°C). Dropwise, inject in the molybdenum (solution 2) over the course of 2-3 hours (note, go slow). After all solution has been injected, allow the mixture to sit just below reflux for 1 more hour. Remove substrates or material from the solution and rinsed with hot acetone and hot ethanol, exposing to air at this point is okay. Remove the substrate and rinse with acetone (25°C).
| Mo(CO)6 | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|
| 0.01-0.03 g | Total = 10 mLs | 3 hours | 2 atm % ± 1% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)