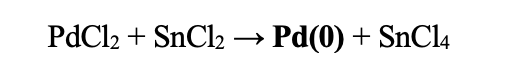
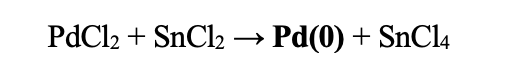

| Redox Potentials: | SnCl2 → SnCl4 | -0.1375 | |
| Pd2+ + 2e- → Pd(0) | 0.951 | ||
| Total: | 1.0885 |
This reaction will favor formation of Pd(0) and SnCl4. The fact that this works was surprising.
There is no need to prepare the glassware before intercalation. Setup the reflux apparatus as shown in Fig 2. There is also no need to use an air-free setup. Prepare a stock solution of stannous chloride from 0.01 g of SnCl2 in 5 mL acetone.
Heat a solution of 0.01 g palladium(II) chloride in 5 mL acetone in the round-bottom flask to just below reflux (48°C). Once heated, drop the 2D material, free or suspended on a substrate, into the round bottom flask. Dropwise, add in the solution of tin(II) chloride very slowly over the course of 3 hours. Keep heating during this time at just below reflux. After 3 hours, and complete injection of the stannous chloride solution, wait an additional 30 – 45 mins with heating. Afterwards, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetone (~45°C) will help remove any other salt impurities that may end up on the substrate.
There is an alternative method that has only barely been explored but shows promise. This one is expensive and perhaps not worth the cost unless you happen to have Pd-DBA sitting around. Palladium may be intercalated through decomposition tris(dibenzylideneacetone) dipalladium(0) [Pd2(DBA)3] decomposition. Dissolve 0.01 g of Pd-DBA in 5 mL of THF. Inject into 5mL of anhydrous octadecene heated to 150°C.
Things that don’t work: Diol reduction of palladium compounds goes too fast resulting in nanoparticle deposits with no intercalation.
| PbCl2 | SnCl2 | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|---|
| 0.01 g | 0.1 g | 5 mL | 4 hours | 1.76 atm % ± 3% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)