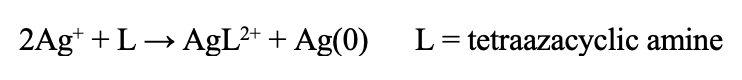
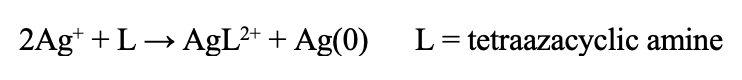

There is no need to prepare the glassware. Setup the reflux setup as shown. There is no need to use an air-free setup. Heat a solution of 0.01 g silver nitrate (AgNO3) and 0.1g 5,5,7,12,12,14-hexamethyl-1,4,8,11- tetraazocyclotetradecane in 5 mL acetonitrile in the round-bottom flask to just below reflux (48°C). Once heated, drop in the 2D material, free or suspended on a substrate, into the round bottom flask. Keep heating just below reflux for 4 hours. After 4 hours, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetontrile or watere will help remove any other salt impurities that may end up on the substrate.
| AgNO3 | Tetroazocyclicamine | acetonitrile | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|---|
| 0.01 g | 0.1 g | 5 mL | 4 hours | 50-60 atm % ± 5% |
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)