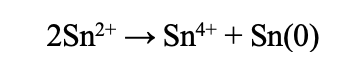
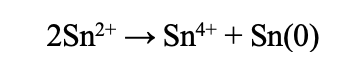

There is no need to prepare the glassware before intercalation. Setup the reflux setup as shown in Fig 2. There is also no need to use an air-free setup. The following instructions assume the usage of tartaric acid.
Prepare a stock solution of stannous chloride and tartrate from both 0.03 g of SnCl2 and 0.03 – 0.06 g of tartaric acid in 10 mL acetone. Thoroughly agitate the mixture until completely dissolved. Heat the solution in the round-bottom flask to just below reflux (48°C). Once heated, drop in the 2D material, free or suspended on a substrate, into the round bottom flask. Keep heating just below reflux for 4 hours. Afterwards, remove the substrate or 2D crystal from solution and rinse with acetone or ethanol several times. Rinsing with heated acetone will help remove any other salt impurities that may end up on the substrate.
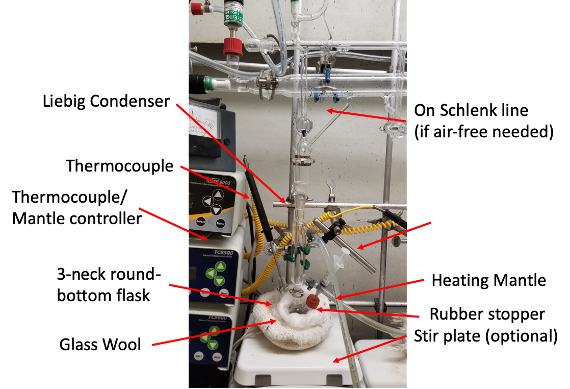
Sn deintercalation should be performed under a nitrogen environment using the Schlenk line technique to avoid hydrolysis of SnCl4. Prepare the setup in Fig 2/3. Place the Sn-intercalated samples into a round bottom flask. Evacuate and flush with N2 several times. Dissolve 0.23 g of SnCl4 in 5 ml anhydrous acetone. Inject the SnCl4 acetone solution into the flask and keep just under reflux (52°C) with N2 flow for 6 hours. This is a slower reaction but heating expedites tin deintercalation. The material is removed from the flask, rinsed with acetone several times, and dried in air after deintercalation.
| SnCl2 | Tartaric Acid | acetone | time | ~ intercalated in Bi2Se3 |
|---|---|---|---|---|
| 0.036 g | 0.03-0.11 g | 10 mL Total | 1 hour | 20 atm % ± 3% |
M. Wang, I. Al-Dhahir, J. Appiah, and K. J. Koski, Deintercalation of zero-valent metals from 2D layered chalcogenides. Chem. Mater. 29, 1650-1655 (2017).
M. Wang, D. Williams, G. Lahti, S. Teshima, D. Dominguez-Aguilar, K. J. Koski, Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 5, 045005 (2018)